Comparative Metabolism Laboratory
 The Comparative Metabolism laboratory investigates glucose metabolism during metabolic
diseases (e.g., diabetes, insulin resistance and obesity) and novel metabolic therapeutic
strategies that could benefit both veterinary and human patients. Diabetes and obesity,
global epidemic diseases, are significant risk factors for
The Comparative Metabolism laboratory investigates glucose metabolism during metabolic
diseases (e.g., diabetes, insulin resistance and obesity) and novel metabolic therapeutic
strategies that could benefit both veterinary and human patients. Diabetes and obesity,
global epidemic diseases, are significant risk factors for
cardiovascular and pulmonary diseases. One of the major pathogenic processes underlying these metabolic diseases results from a deficit of insulin production or action, with dysfunctional glucose uptake into insulin-sensitive tissues.
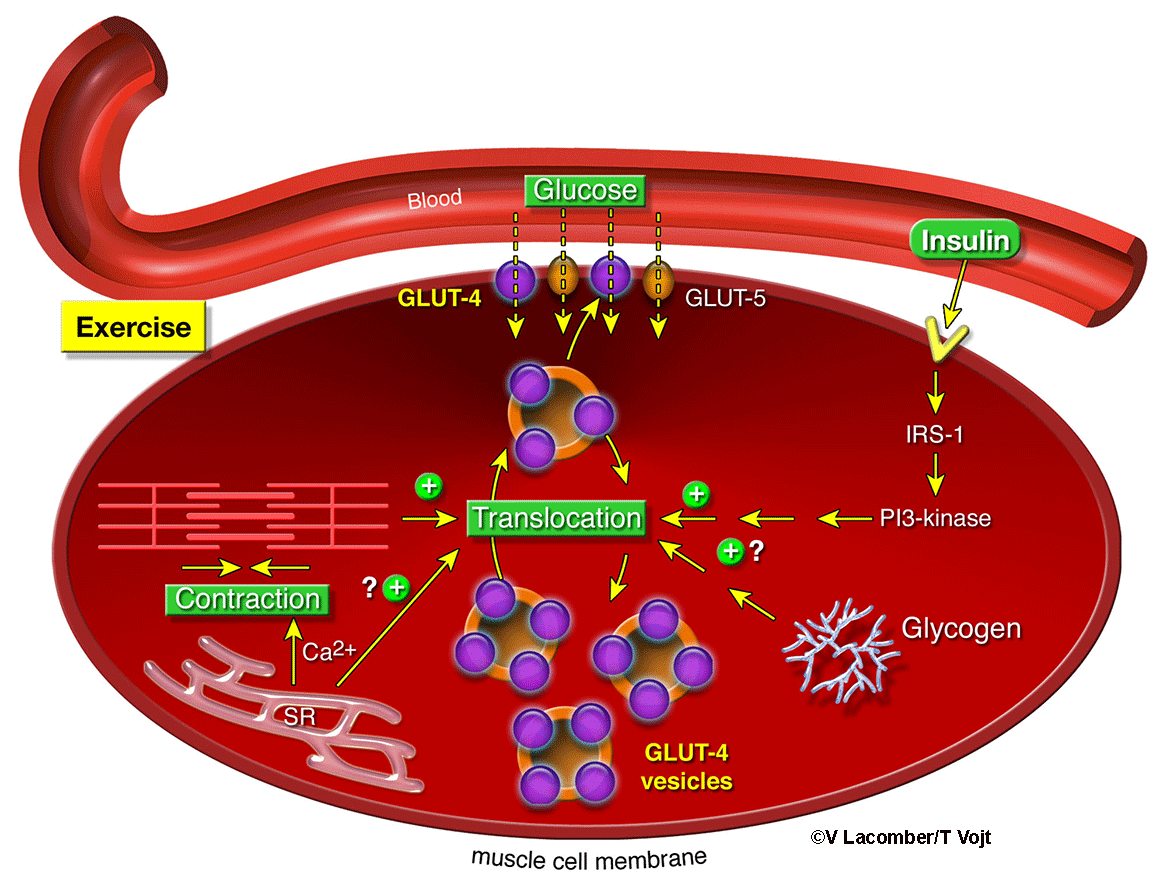 Despite intensive research, the mechanisms of altered glucose transport observed during
diabetes are still not well elucidated. Therefore, the main current mission of our
research laboratory is to investigate the regulation of glucose transport in insulin-sensitive
tissues (e.g., cardiac and skeletal muscles, and adipose tissues), but also in the
lung and lamellar tissue. Glucose uptake from the bloodstream is tightly regulated
by a family of specialized proteins called glucose transporters. Because every cell
expresses these glucose transporters, they are recognized as major regulators of whole-body
metabolism and thus, key pharmacological targets. The strength of our research program
is to use a unique comparative approach by investigating both small and large animal
models of metabolic diseases, spanning from transgenic mice to horses.
Despite intensive research, the mechanisms of altered glucose transport observed during
diabetes are still not well elucidated. Therefore, the main current mission of our
research laboratory is to investigate the regulation of glucose transport in insulin-sensitive
tissues (e.g., cardiac and skeletal muscles, and adipose tissues), but also in the
lung and lamellar tissue. Glucose uptake from the bloodstream is tightly regulated
by a family of specialized proteins called glucose transporters. Because every cell
expresses these glucose transporters, they are recognized as major regulators of whole-body
metabolism and thus, key pharmacological targets. The strength of our research program
is to use a unique comparative approach by investigating both small and large animal
models of metabolic diseases, spanning from transgenic mice to horses.
Research
-
Glucose Metabolism in the Heart
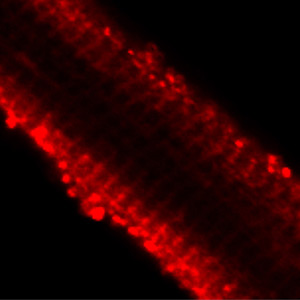 GLUT4 protein at the cell surface of cardiac myocytes upon insulin treatment (Method:
confocal laser scanning microscopy).Diabetes has been identified as a major risk factor for cardiovascular diseases, such
as diabetic cardiomyopathy, heart failure and atrial fibrillation (the most common
cardiac arrhythmia). Although the heart is a major organ to utilize glucose, the regulation
of glucose transport in the heart remains not well elucidated, especially in regard
to the insulin-independent pathways. To evaluate GLUT trafficking, we have established
a biotinylated photolabeling technique that provides a direct quantifiable measure
of active cell surface GLUTs. Using this state-of-the-art technique, the Comparative
Metabolism Laboratory demonstrated that sarcoplasmic reticulum calcium handling, which
tightly regulates excitation-contraction coupling, is a major regulator of glucose
transport during healthy and insulin-deficient state. We also characterized the function
of some novel GLUT isoforms (mostly in the class 3). Our laboratory also showed that
insulin dysregulation and alterations in glucose transport are major contributors
to atrial fibrillation during diabetes.
GLUT4 protein at the cell surface of cardiac myocytes upon insulin treatment (Method:
confocal laser scanning microscopy).Diabetes has been identified as a major risk factor for cardiovascular diseases, such
as diabetic cardiomyopathy, heart failure and atrial fibrillation (the most common
cardiac arrhythmia). Although the heart is a major organ to utilize glucose, the regulation
of glucose transport in the heart remains not well elucidated, especially in regard
to the insulin-independent pathways. To evaluate GLUT trafficking, we have established
a biotinylated photolabeling technique that provides a direct quantifiable measure
of active cell surface GLUTs. Using this state-of-the-art technique, the Comparative
Metabolism Laboratory demonstrated that sarcoplasmic reticulum calcium handling, which
tightly regulates excitation-contraction coupling, is a major regulator of glucose
transport during healthy and insulin-deficient state. We also characterized the function
of some novel GLUT isoforms (mostly in the class 3). Our laboratory also showed that
insulin dysregulation and alterations in glucose transport are major contributors
to atrial fibrillation during diabetes. -
Glucose Metabolism in the Lung
The CDC has recently identified that diabetic patients are three times more likely to die from influenza or pneumonia as their non-diabetic counterparts. This phenomenon is thought to occur due to the hyperglycemic state predisposing patients to a higher-than-normal glucose concentration in the airway, leading to an increased risk of pulmonary infections. Although the lung is a major organ to utilize glucose, the role and the regulation of glucose homeostasis in the lung have received little attention. Therefore, our long-term goal is to investigate the role and alterations of glucose homeostasis that occur during respiratory infections.
-
Glucose Metabolism during Equine Metabolic Syndrome and Laminitis
Similar to humans, metabolic disease is also increasingly diagnosed in veterinary patients, with equine obesity and insulin resistance burgeoning. However, the pathogenesis underlying equine metabolic syndrome is not well known and studies from the Comparative Metabolism Laboratory provided some novel mechanistic insights into this insidious disease. For instance, we demonstrated a novel link between inflammation and impaired glucose metabolism. The Comparative Metabolism Laboratory is also investigating the pathogenesis of equine laminitis, a debilitating foot disease, which occurs during Equine Metabolic Syndrome. Although insulin resistance is at the forefront of current investigations of laminitis, the role of insulin resistance and impaired glucose transport in the foot is not well known.
-
Grants (selected)
- NIH P20 GM103648 (Lacombe, Project leader). The role of glucose homeostasis during respiratory infections.
- NIH 7P20GM10493 (Pilot Project). The SERCA pump as a major regulator of glucose metabolism.
- NIH P20 GM103648 (Pilot Project). Regulation of glucose transport in the healthy and diabetic lung.
- NIH K01RR023083. Impaired glucose transport during diabetes: the novel role of calcium.
- Acorda Therapeutics Research Award. The role of Glial Growth Factor 2 as a regulator of glucose transport in the healthy and failing myocardium.
- American Heart Association (GIA 0425361B). Novel mechanisms of diastolic dysfunction in the diabetic myocardium
- Harold Hamm Diabetes Center Enrichment Research Award. The regulation of glucose transport in the atria: Impact during diabetes.
- United States Equestrian Federation. Investigation of glucose transport pathways in an equine model of insulin resistance.
